 400-9988-900
400-9988-900
 400-9988-900
400-9988-900
Preface
The pharmaceutical industry is a supporting industry for human health. With the intensification of the aging of the global population and the outbreak of Covid-19 of public safety emergency event, human demand for medicine continues to grow. API is the foundation of pharmaceutical products. The increase in demand for pharmaceuticals has brought an increase in the demand for APIs, which has then caused an increase in labor demand. However, the aging society keeps our working-age population decreasing. Therefore, the upgrade of production capacity for API manufacturing is imperative, which can be achieved by improving automation to reduce the demand for manual workers.
Sterile APIs are APIs prepared through aseptic processes. The preparation of “Non-terminally sterilized” aseptic APIs not only requires labor force, but also requires skilled people who are trained and qualified, and subject to management. EU-GMP appendix 1 "Production of Sterile Drugs" emphasizes that pharmaceutical companies need to develop and continuously improve pollution control strategies. Human-beings are the largest source of pollution in aseptic processes, for which Truking has established pollution control strategies and its own capacity of continuous technical improvements for freeze-dried sterile APIs, through long-term practice in the production of freeze-dried sterile APIs.
Sources of Contamination Risks in Pharmaceutical Production
|
Source
|
Pollution risk |
|
Plant
|
Unreasonable personnel and material flow lead to mixing up and pollution |
|
Air filtration system
|
Moisture or dust pollution caused by the number of air changes, air supply, air return and air exhaust. |
|
Process equipment
|
Process and equipment do not match each other, leading to decrease of production capacity and quality
|
|
personnel |
Factors such as health level, professionalism, character habits and professional skills affect the whole process of drug production а |
system design
Based on the Qbd principle, good production practice comes from good system design. The open manufacturing mode of traditional freeze-dried APIs is an important factor that causes many pollution risks. Therefore, changing the open system to a closed system is the first solution to control pollution.
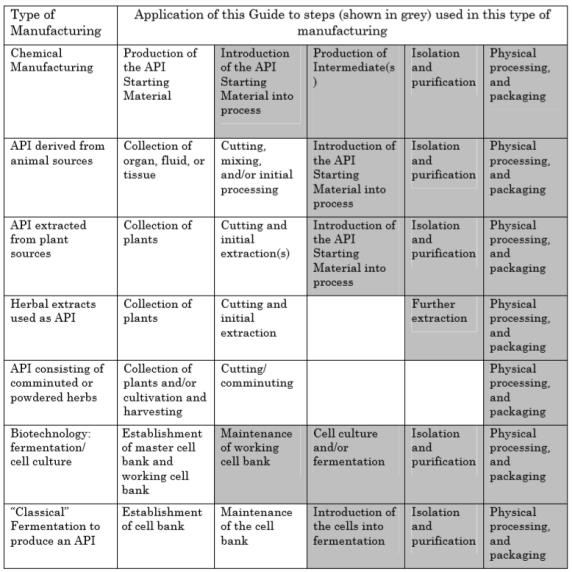
Figure 1: Application of this Guide to API Manufacturing(ICH Q7a)
Process layout design
A complete freeze-dried sterile API workshop mainly includes (but is not limited to) preparation tanks, filters, loading devices, vacuum freeze dryer, unloading devices, CIP station, pulverizer, blender, filling machine, capping machine and isolator etc.
Key equipment design
Freeze dryer
the freeze dryer has folded edges around shelf, which has the function of directly containing liquid medicine, reducing tools used in aseptic area;
Shelf is equipped with discharge port. When all shelves are descending, the discharge port is automatically opened to drain cleaning water inside shelf and ensure shelves are cleaned thoroughly;
Shelf can rise and fall, and has the functions of equal height product filling and equal height product unloading;
The loading device is integrated into the freeze dryer, and the telescopic loading function of each shelf can be realized through movement of shelf;
The freeze dryer is equipped with an automatic slot door, and the product unloading is carried out by opening the slot door to reduce dust exposure area;
The unloading device is fully automatic, reducing human operation;
Product unloading with equal height, small displacement in vertical direction of equipment, with low height of the device; uniform wind speed of unidirectional flow system of entire unloading working surface and within controllable range of guide value, easy to verify, and no additional scientific basis is required;
Isolator
The isolator is highly integrated with unloading door, unloading device, and aseptic filling device of the freeze dryer, and the core working surface of unloading operation is placed under unidirectional flow protection in the isolator;
The isolator is equipped with independent air-conditioning system to control air volume and temperature and humidity of aseptic core area to completely eliminate influence of plant facilities on aseptic core area;
The barrier system of isolator completely eliminates unintended manual intervention, ensuring that the behavior of operators in process is controllable and operators are completely isolated;
the Isolator has its own cleaning system, and the disinfection system ensures the cleanliness and sterility in equipment.
Risk control strategy
|
Source |
Risk control strategy |
|
Plant |
Process and equipment are highly integrated, and aseptic core area is achieved through isolators and automation |
|
Air filtration system |
Separate air-conditioning system for aseptic core area |
|
Process equipment |
Matching of process and equipment can reduce risk of drug manufacturing process |
|
personnel |
Isolate operators from sterile core area |
Good design is the prerequisite to guarantee implementation of aseptic process and product quality. Truking will continue to improve intelligent level of freeze-dried aseptic API manufacturing through continuous pharmaceutical engineering practice.
Reference document
Good Manufacturing Practice for Drugs (Revised in 2010)
Pharmaceutical GMP Guidelines: Sterile Preparation
EU GMP Annex 1:Manufacture of Sterile Products
ICH Q7a GMP Guidance for APIs
